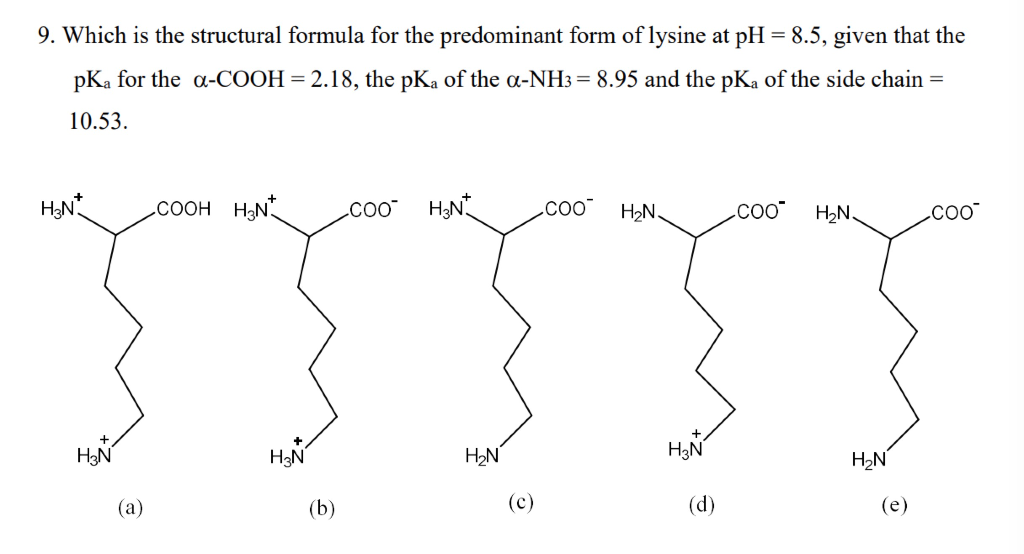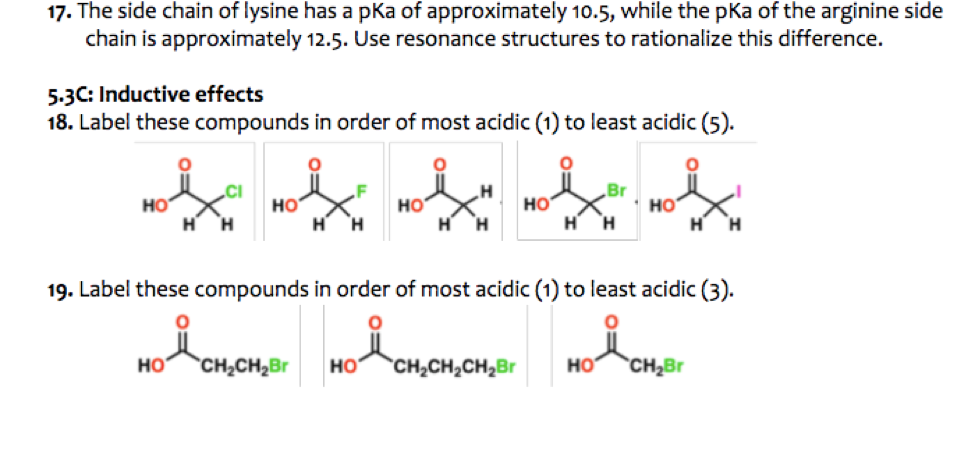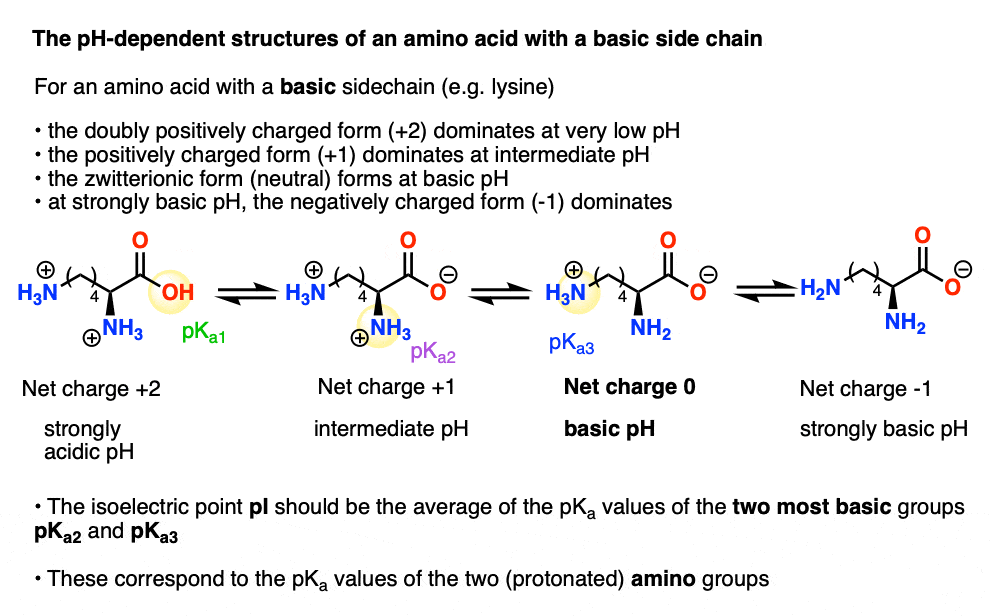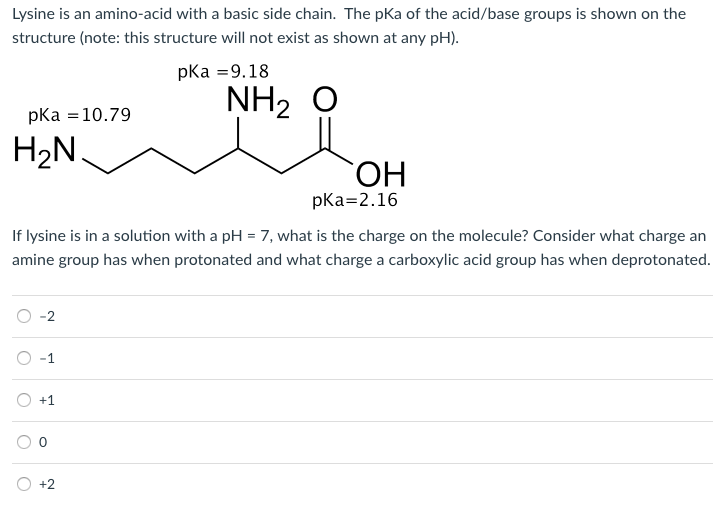
What are the reasons these Amino Acid "side-chain R groups" do not have a pKa? No pKa means that hydrogens can neither leave or attach to these amino acids, right? : r/chemhelp

Impact of Side Chain Polarity on Non-Stoichiometric Nano-Hydroxyapatite Surface Functionalization with Amino Acids | Scientific Reports

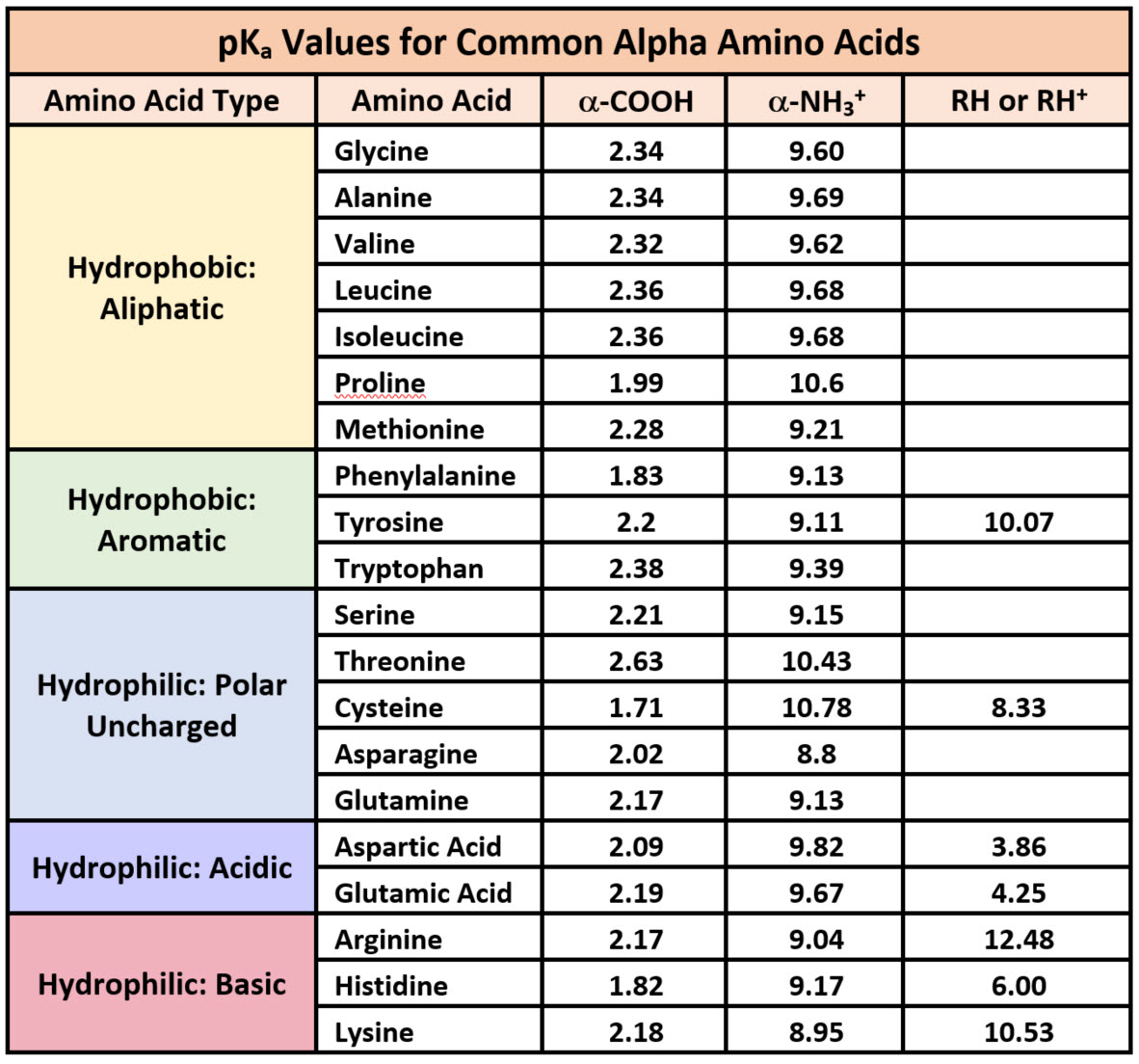
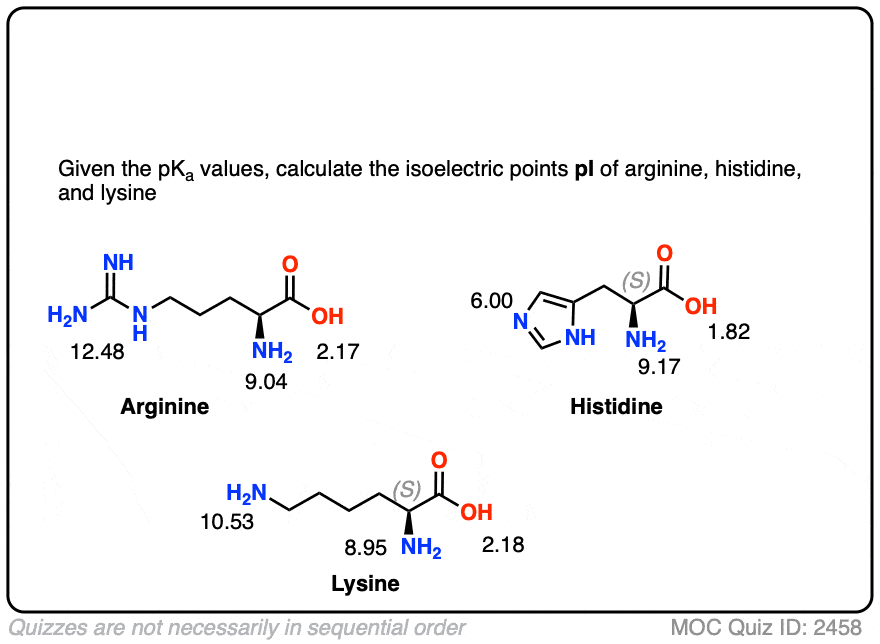
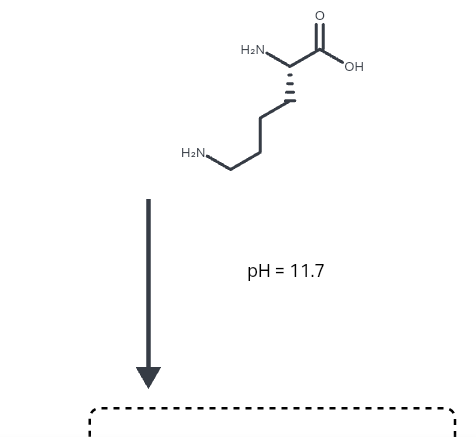
All amino acids have two ionizable groups (an alpha-amino group with pKa = 9.3, and an alpha-carboxyl group with pKa = 2.2). Lysine also has an ionizable side-chain (R) with a pKa

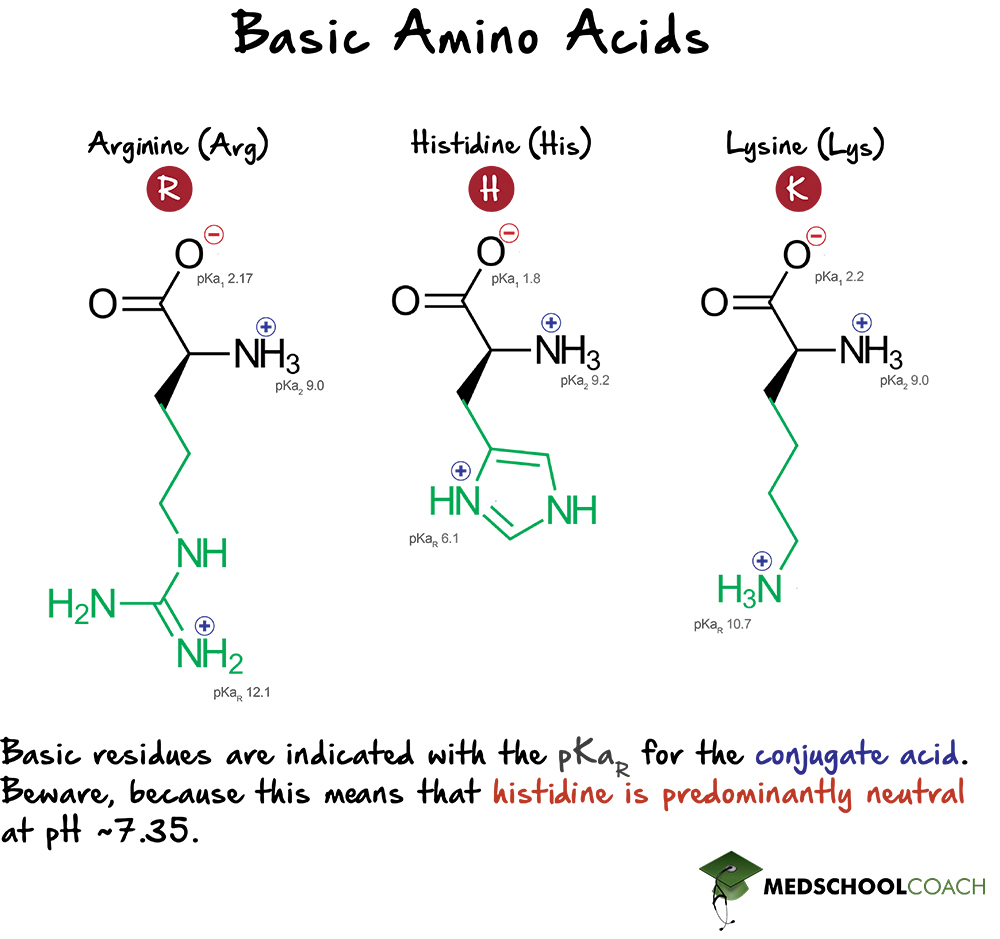
Structure of common basic and acidic amino acids, with the pKa values... | Download Scientific Diagram
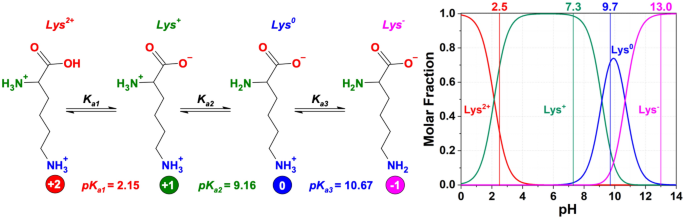
Modulating the poly-l-lysine structure through the control of the protonation–deprotonation state of l-lysine | Scientific Reports