
Spinal Cord Stimulation for Painful Diabetic Neuropathy - Andrea M. Yeung, Jingtong Huang, Kevin T. Nguyen, Nicole Y. Xu, Lorenzo T. Hughes, Brajesh K. Agrawal, Niels Ejskjaer, David C. Klonoff, 2024
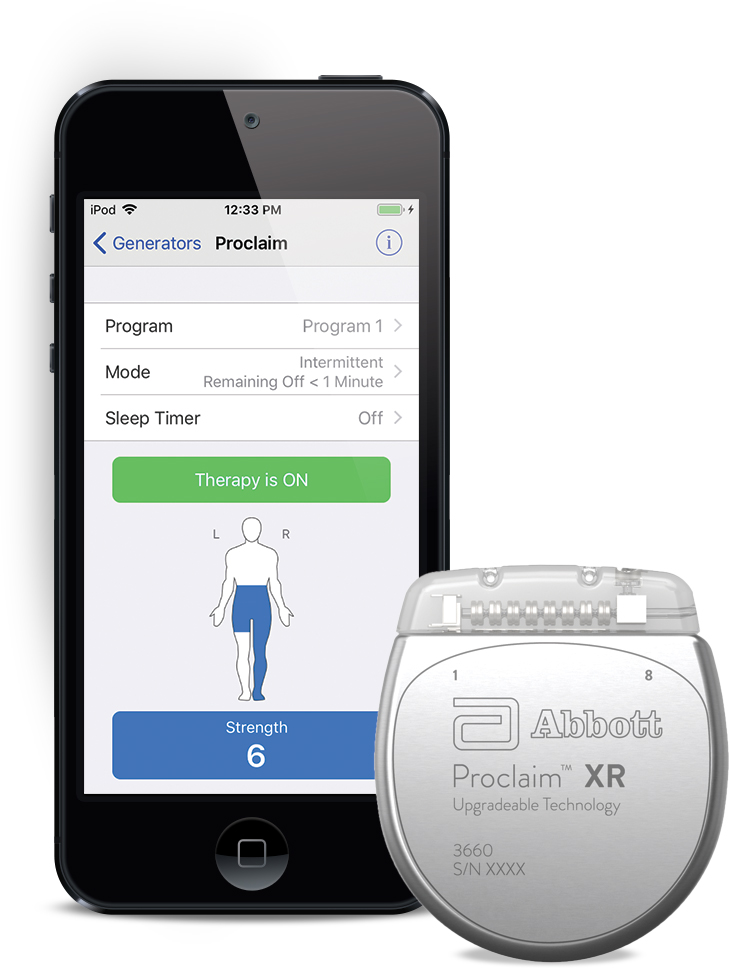
FDA Approves Abbott's "Low Dose," Recharge-Free Spinal Cord Stimulation System with up to Ten Year Battery Life* for People Living with Chronic Pain - Sep 26, 2019

alexander escobar, m.d. on X: "Alongside the nations leading experts in pain medicine to implant the worlds first ever @AbbottGlobal recharge free ProclaimXR spinal cord stimulator for the treatment of chronic pain.

















